Introduction to physical and chemical changes worksheet answers provides a comprehensive exploration of the fundamental concepts surrounding physical and chemical changes. This guide delves into the intricacies of these transformations, equipping readers with a thorough understanding of their distinct characteristics, practical applications, and safety considerations.
Physical changes involve alterations in the form or appearance of a substance without modifying its chemical composition. Examples include melting, freezing, and boiling. Chemical changes, on the other hand, result in the formation of new substances with different chemical properties.
Burning, rusting, and digestion are common examples of chemical changes.
Physical Changes
Physical changes are changes in the form or appearance of a substance without changing its chemical composition. They involve changes in the physical properties of the substance, such as its shape, size, or state.
Examples of physical changes include melting, freezing, boiling, sublimation, and condensation. Melting is the process of a solid turning into a liquid, while freezing is the reverse process of a liquid turning into a solid. Boiling is the process of a liquid turning into a gas, while condensation is the reverse process of a gas turning into a liquid.
Sublimation is the process of a solid turning directly into a gas, bypassing the liquid state.
Physical changes are typically reversible, meaning that the original substance can be restored by reversing the change. For example, ice can be melted into water and then frozen back into ice. This reversibility is due to the conservation of mass, which states that the total mass of the system remains the same throughout the change.
Chemical Changes
Chemical changes, also known as chemical reactions, are changes in the chemical composition of a substance. They involve the formation of new substances with different properties from the original substance.
Examples of chemical changes include burning, rusting, and digestion. Burning is the process of a substance reacting with oxygen to produce heat and light. Rusting is the process of iron reacting with oxygen and water to form iron oxide. Digestion is the process of breaking down food into smaller molecules that can be absorbed by the body.
Chemical changes are typically irreversible, meaning that the original substance cannot be restored by reversing the change. For example, once wood is burned, it cannot be turned back into wood. This irreversibility is due to the formation of new substances with different chemical compositions.
Identifying Physical and Chemical Changes
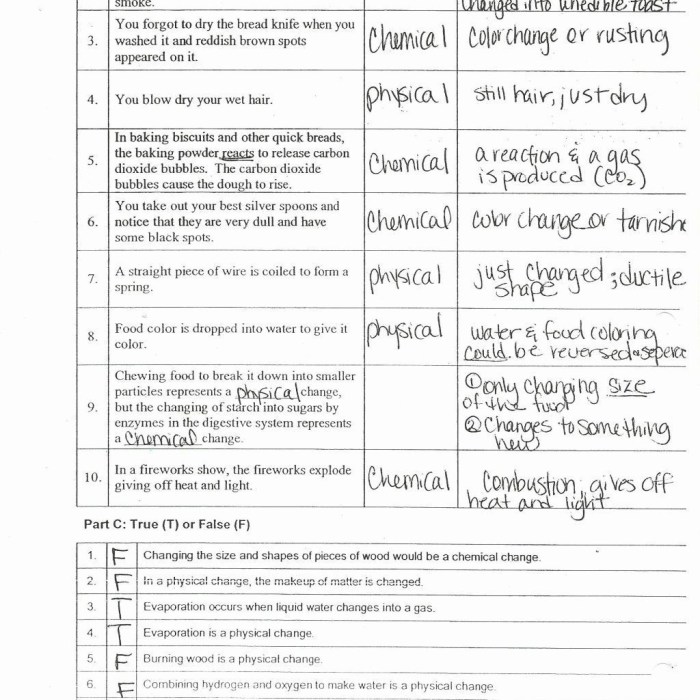
The following table provides a list of examples and classifies each one as a physical or chemical change:
| Physical Change | Chemical Change |
|---|---|
| Melting ice | Burning wood |
| Freezing water | Rusting iron |
| Boiling water | Digestion of food |
| Condensation of water vapor | Photosynthesis |
| Sublimation of dry ice | Respiration |
The criteria used to differentiate between physical and chemical changes include:
- Whether or not the change is reversible
- Whether or not the change produces new substances
- Whether or not the change releases or absorbs energy
Applications of Physical and Chemical Changes
Physical changes have many practical applications, such as in cooking, refrigeration, and materials science.
- In cooking, physical changes are used to change the texture and flavor of food. For example, cooking meat causes it to change from a tough, fibrous texture to a tender, juicy texture.
- In refrigeration, physical changes are used to keep food fresh. For example, freezing food slows down the growth of bacteria and other microorganisms.
- In materials science, physical changes are used to create new materials with desired properties. For example, annealing metals makes them stronger and more durable.
Chemical changes also have many practical applications, such as in medicine, energy production, and manufacturing.
- In medicine, chemical changes are used to create drugs and other medical treatments. For example, antibiotics are used to kill bacteria, and vaccines are used to prevent diseases.
- In energy production, chemical changes are used to generate electricity. For example, burning fossil fuels releases energy that can be used to turn turbines and generate electricity.
- In manufacturing, chemical changes are used to create a wide variety of products, such as plastics, fertilizers, and paper.
Safety Considerations: Introduction To Physical And Chemical Changes Worksheet Answers
Physical and chemical changes can be hazardous, so it is important to take precautions when working with them.
- Physical changes can cause burns, cuts, and other injuries. For example, hot liquids can cause burns, and sharp objects can cause cuts.
- Chemical changes can release toxic gases, fumes, and other hazardous substances. For example, burning wood releases carbon monoxide, which is a poisonous gas.
To minimize the risks associated with physical and chemical changes, it is important to follow these safety guidelines:
- Wear appropriate protective gear, such as gloves, goggles, and a lab coat.
- Work in a well-ventilated area.
- Handle chemicals with care and follow the manufacturer’s instructions.
- Dispose of chemicals properly according to local regulations.
Common Queries
What are the key differences between physical and chemical changes?
Physical changes alter the form or appearance of a substance without changing its chemical composition, while chemical changes result in the formation of new substances with different chemical properties.
How can we identify whether a change is physical or chemical?
Physical changes are typically reversible and do not involve the formation of new substances, whereas chemical changes are irreversible and result in the creation of new substances with different properties.
What are some practical applications of physical and chemical changes?
Physical changes are used in various processes such as cooking, refrigeration, and materials science, while chemical changes find applications in medicine, energy production, and manufacturing.