How many molecules are in a mole of caffeine? This seemingly straightforward question opens the door to a fascinating journey into the realm of chemistry and measurement. Delving into the depths of Avogadro’s number, molecular weight, and the very definition of a mole, we embark on a quest to unravel the intricacies of this essential concept.
Unveiling the structure of caffeine, we dissect its chemical formula and functional groups, gaining insights into its molecular makeup. By meticulously calculating its molecular weight, we establish a bridge between the microscopic and macroscopic worlds, paving the way for determining the number of molecules in a mole.
Mole Definition
A mole is a fundamental unit of measurement in chemistry. It is defined as the amount of substance that contains exactly 6.02214076 × 10 23elementary entities.
The elementary entity can be an atom, molecule, ion, or electron. The mole concept is based on Avogadro’s number, which is the number of atoms in 12 grams of carbon-12.
Caffeine Structure
Caffeine has the chemical formula C 8H 10N 4O 2.
Its molecular structure consists of a purine ring system with three methyl groups attached. The purine ring system is composed of two fused five-membered rings, and the methyl groups are located at positions 1, 3, and 7.
Molecular Weight
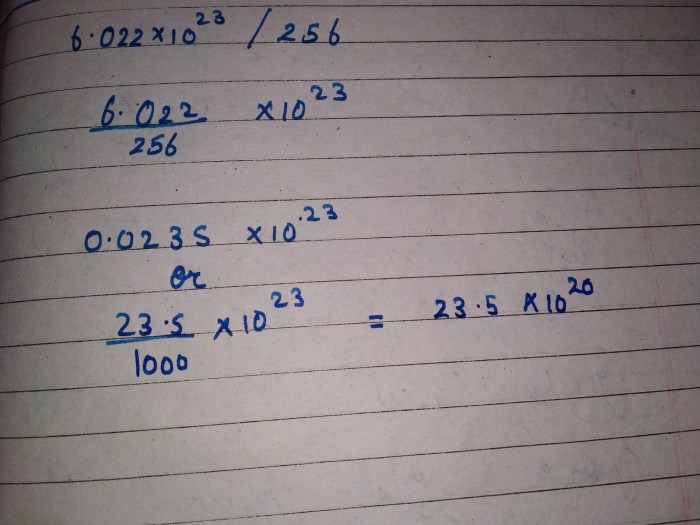
The molecular weight of caffeine is calculated by adding the atomic weights of each element in its chemical formula.
- 8 carbon atoms: 8 × 12.01 = 96.08
- 10 hydrogen atoms: 10 × 1.01 = 10.10
- 4 nitrogen atoms: 4 × 14.01 = 56.04
- 2 oxygen atoms: 2 × 16.00 = 32.00
Molecular weight of caffeine = 96.08 + 10.10 + 56.04 + 32.00 = 194.22 g/mol
Avogadro’s Number
Avogadro’s number is 6.02214076 × 10 23. It represents the number of elementary entities present in one mole of a substance.
This number is used to convert between the mass and the number of molecules in a substance.
Calculating Molecules in a Mole: How Many Molecules Are In A Mole Of Caffeine
The number of molecules in a mole of a substance can be calculated using the following formula:
Number of molecules = Number of moles × Avogadro’s number
For caffeine, the number of molecules in a mole can be calculated as follows:
Number of molecules = 1 mole × 6.02214076 × 10 23molecules/mole = 6.02214076 × 10 23molecules
Applications
Knowing the number of molecules in a mole of caffeine has several practical applications in chemistry and other fields.
- Determining the concentration of caffeine solutions
- Calculating the amount of caffeine in a given sample
- Understanding the physiological effects of caffeine
- Designing new drugs and therapies that target caffeine receptors
FAQ Explained
What is the significance of Avogadro’s number?
Avogadro’s number serves as a fundamental constant, representing the number of atoms, molecules, or ions present in one mole of a substance. It enables us to establish a direct link between the macroscopic and microscopic scales, facilitating precise measurements and calculations in chemistry.
How is the molecular weight of caffeine calculated?
The molecular weight of caffeine is determined by summing the atomic weights of each element present in its molecular formula. For caffeine, with the formula C8H10N4O2, the molecular weight is calculated as (8 x 12.01) + (10 x 1.01) + (4 x 14.01) + (2 x 16.00) = 194.19 g/mol.